What Is Density Of Water G Cm3? Easy Calculation
The density of water is a fundamental physical property that is essential in various fields, including chemistry, physics, and engineering. At its core, density is defined as mass per unit volume of a substance. For water, this value is crucial for understanding its behavior under different conditions.
To calculate the density of water, we need to know its mass and volume. The standard unit of density is grams per cubic centimeter (g/cm³) or kilograms per liter (kg/L), with 1 g/cm³ being equivalent to 1 kg/L. The density of water is approximately 1 gram per milliliter (g/mL) or 1 gram per cubic centimeter (g/cm³) at standard temperature and pressure conditions.
Calculation
The calculation of density is straightforward and can be represented by the formula:
[ \text{Density} = \frac{\text{Mass}}{\text{Volume}} ]
For water, if we have 1 gram of water, and its volume is 1 milliliter (mL) or 1 cubic centimeter (cm³), then the density can be calculated as follows:
[ \text{Density of Water} = \frac{1 \, \text{gram}}{1 \, \text{cm}^3} = 1 \, \text{g/cm}^3 ]
This shows that 1 gram of water occupies a volume of 1 cubic centimeter, hence the density of water is 1 g/cm³.
Factors Affecting Density
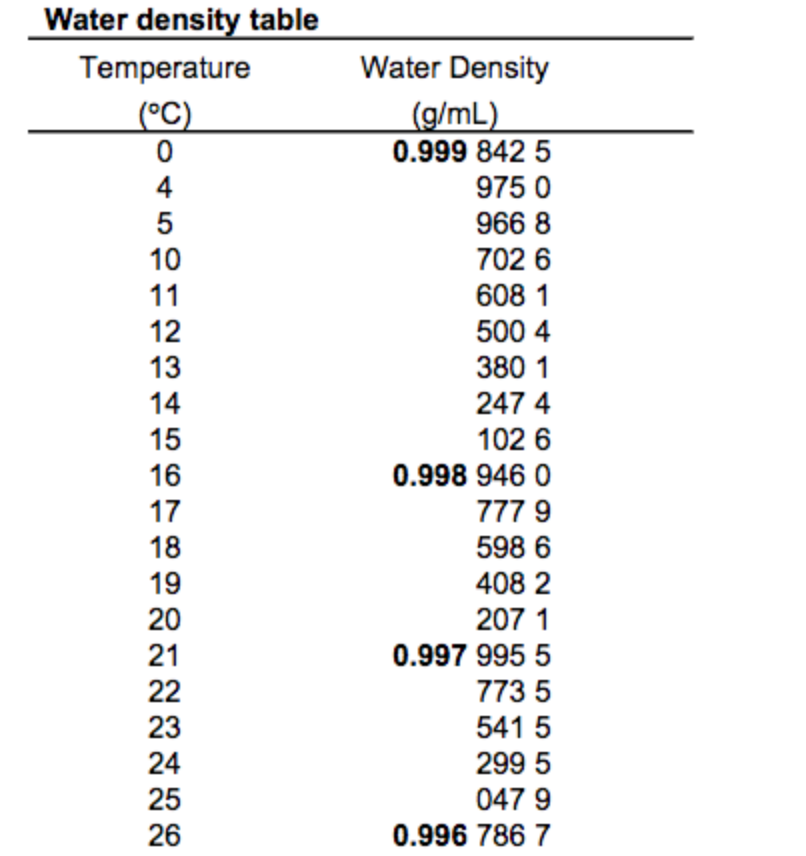
It’s worth noting that the density of water can vary slightly depending on temperature and pressure. Pure water at standard conditions (20°C or 68°F and 1 atmosphere of pressure) has a density very close to 1 g/cm³. However, as the temperature increases, the density of water decreases, reaching its minimum at around 4°C, where it is approximately 0.99997 g/cm³, before increasing again as it freezes into ice.
This unique property of water, where it expands when it freezes (unlike most substances which contract), is why ice floats on liquid water, a phenomenon critical for many biological and environmental processes.
Practical Applications
Understanding the density of water is crucial in various practical applications:
Buoyancy Calculations: Knowing the density of water is essential for calculating the buoyancy force on objects submerged in water, which is important in naval architecture, off-shore engineering, and even in the design of underwater vehicles.
Water Quality Assessment: Changes in water density can indicate variations in the concentration of dissolved substances, making it a parameter in water quality monitoring.
Hydroelectric Power: The density of water influences the pressure it exerts with depth, a principle utilized in hydroelectric power generation.
Biological Systems: The density of water affects the floating and sinking of biological organisms and objects in aquatic ecosystems, influencing habitats and distribution patterns.
Conclusion
In conclusion, the density of water, approximately 1 g/cm³ at standard conditions, is a fundamental property with significant implications across various scientific disciplines and practical applications. Its calculation is straightforward, yet its variations with temperature and pressure underscore the complexity of water’s physical properties and their importance in both natural and engineered systems.
What is the standard density of water in g/cm³?
+The standard density of water is approximately 1 gram per cubic centimeter (g/cm³) at standard temperature and pressure conditions.
How does temperature affect the density of water?
+The density of water decreases as the temperature increases, reaching a minimum at around 4°C, before increasing again as it freezes into ice.
What are some practical applications of knowing the density of water?
+Some practical applications include buoyancy calculations, water quality assessment, hydroelectric power generation, and understanding biological systems in aquatic ecosystems.


