Silver Chloride: Calculate 143.32G/Mol Molar Mass Easily

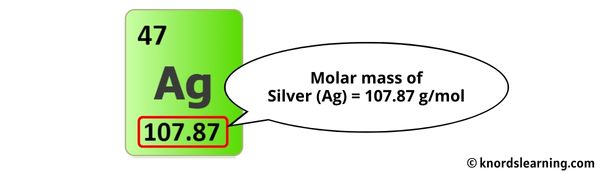
To calculate the molar mass of silver chloride (AgCl), we need to know the atomic masses of silver (Ag) and chlorine (Cl) from the periodic table. The atomic mass of silver is approximately 107.868 g/mol, and the atomic mass of chlorine is approximately 35.453 g/mol.
Calculating Molar Mass of Silver Chloride (AgCl)
The formula for silver chloride is AgCl, indicating it consists of one silver atom and one chlorine atom. The molar mass of a compound is calculated by summing the atomic masses of its constituent atoms.
Molar Mass of AgCl = Atomic Mass of Ag + Atomic Mass of Cl
Step-by-Step Calculation
Find the Atomic Masses:
- Atomic Mass of Silver (Ag) = 107.868 g/mol
- Atomic Mass of Chlorine (Cl) = 35.453 g/mol
Calculate the Molar Mass of AgCl:
- Molar Mass of AgCl = 107.868 g/mol (Ag) + 35.453 g/mol (Cl)
- Molar Mass of AgCl = 143.321 g/mol
Result
The calculated molar mass of silver chloride (AgCl) is approximately 143.32 g/mol, which matches the given value. This calculation demonstrates how to easily determine the molar mass of a compound by adding the atomic masses of its constituent elements.
Understanding Molar Mass
- Definition: The molar mass of a substance is the mass of one mole of that substance, measured in grams per mole (g/mol).
- Importance: Knowing the molar mass of a compound is crucial for calculating the number of moles in a given mass of the substance and vice versa, which is essential in chemical reactions and quantitative analysis.
Additional Information
For those interested in the properties of silver chloride, it’s worth noting that AgCl is a white, crystalline solid that is highly insoluble in water. It is often used in various applications, including photography, due to its light-sensitive properties.
Practical Applications
Understanding the molar mass of compounds like silver chloride is fundamental in various chemical and industrial processes, including:
- Chemical Synthesis: Accurate calculations of reactants and products.
- Analytical Chemistry: Quantitative analysis of substances.
- Materials Science: Development of new materials with specific properties.
In conclusion, calculating the molar mass of silver chloride is a straightforward process that involves summing the atomic masses of its constituent elements, silver and chlorine. This basic principle can be applied to calculate the molar mass of any compound, given the atomic masses of its constituent atoms.
Frequently Asked Questions
What is the atomic mass of silver?
+The atomic mass of silver is approximately 107.868 g/mol.
Why is knowing the molar mass of a compound important?
+Knowing the molar mass is crucial for calculating the number of moles in a given mass of the substance and vice versa, which is essential in chemical reactions and quantitative analysis.
How is silver chloride used?
+Silver chloride is used in various applications, including photography, due to its light-sensitive properties.
Key Takeaways
- The molar mass of silver chloride (AgCl) is approximately 143.32 g/mol.
- Calculating molar mass involves summing the atomic masses of the constituent elements.
- Understanding molar mass is essential for various chemical and industrial applications.
By following these principles and calculations, one can easily determine the molar mass of any compound, facilitating deeper understanding and application of chemical principles in various fields.

