Ir Spectrum Of Ketone: Identify Functional Groups
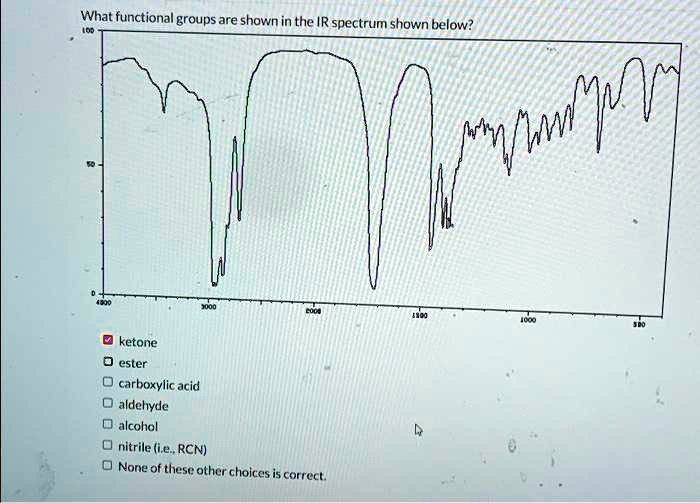
The identification of functional groups in a molecule is a crucial aspect of organic chemistry, and infrared (IR) spectroscopy is a powerful tool for achieving this. Among various functional groups, ketones are significant due to their widespread presence in organic compounds and their role in numerous chemical reactions. The IR spectrum of a ketone provides valuable information about its structure, particularly in identifying the carbonyl group (C=O), which is characteristic of ketones.
Introduction to IR Spectroscopy
IR spectroscopy is based on the principle that molecules absorb specific frequencies of infrared radiation when they vibrated at the same frequency. Different bonds in a molecule vibrate at different frequencies, leading to absorption of radiation at specific wavenumbers (typically measured in cm^-1). The resulting IR spectrum is a plot of the percentage of transmittance versus wavenumber, showing peaks at wavenumbers where absorption occurs. These peaks can be correlated with specific types of bonds or functional groups within the molecule.
Characteristics of Ketone IR Spectra
Ketones are characterized by a strong absorption band due to the carbonyl (C=O) stretch. This band is typically found between 1710 and 1750 cm^-1, though its exact position can vary slightly depending on the ketone’s structure and the solvent used for the IR measurement. For instance:
- Simple Ketones: Unconjugated, simple ketones usually exhibit a C=O stretch absorption around 1715 cm^-1.
- Conjugated Ketones: When a ketone is conjugated with another carbonyl group, an alkene, or an aryl group, the C=O stretching frequency decreases due to resonance stabilization of the carbonyl group, shifting the absorption band to lower wavenumbers (around 1680-1700 cm^-1).
- Cyclic Ketones: The C=O absorption of cyclic ketones can be affected by the ring size. Smaller rings tend to increase the frequency of the C=O stretch due to increased ring strain, while larger rings may decrease it.
Interpreting the IR Spectrum of a Ketone
When interpreting the IR spectrum of a compound suspected to be a ketone, look for the following features:
- C=O Stretch: The most diagnostic feature for a ketone is the strong absorption band corresponding to the C=O stretch, usually found in the region of 1710-1750 cm^-1.
- C-H Stretch: If the ketone has alkyl groups attached, you will see C-H stretching absorptions in the 2850-3000 cm^-1 region.
- C-H Bending: Absorptions due to C-H bending can be seen in the 1350-1500 cm^-1 range.
- Other Functional Groups: The presence of other functional groups can be indicated by additional absorption bands. For example, a broad O-H stretch around 3400 cm^-1 might suggest the presence of an alcohol group, while a sharp peak around 3300 cm^-1 could indicate an amine.
Challenges and Considerations
While IR spectroscopy is a valuable tool for identifying ketones and other functional groups, there are challenges and considerations:
- Overlapping Peaks: In complex molecules, absorption bands from different functional groups can overlap, making interpretation more challenging.
- Weak Absorptions: Some functional groups may exhibit weak absorptions, requiring careful examination of the spectrum or the use of other spectroscopic techniques for confirmation.
- Instrumental Limitations: The quality of the IR spectrum can be affected by the instrument used, the sample preparation, and the solvent choice, if applicable.
Conclusion
The IR spectrum of a ketone is characterized by a strong C=O stretch absorption, typically found between 1710 and 1750 cm^-1. Understanding the nuances of IR spectroscopy and how different structural features affect the spectrum is essential for accurately identifying ketones and other functional groups in organic compounds. By combining IR spectroscopy with other analytical techniques, such as nuclear magnetic resonance (NMR) spectroscopy and mass spectrometry (MS), chemists can gain a comprehensive understanding of a molecule’s structure and properties.
What is the typical range for the C=O stretch absorption in the IR spectrum of a ketone?
+The typical range for the C=O stretch absorption in the IR spectrum of a ketone is between 1710 and 1750 cm^-1.
How does conjugation affect the C=O stretching frequency in ketones?
+Conjugation with another carbonyl group, an alkene, or an aryl group decreases the C=O stretching frequency due to resonance stabilization, shifting the absorption band to lower wavenumbers (around 1680-1700 cm^-1).
What other absorptions might be seen in the IR spectrum of a ketone besides the C=O stretch?
+Besides the C=O stretch, the IR spectrum of a ketone might show C-H stretching absorptions (2850-3000 cm^-1) and C-H bending absorptions (1350-1500 cm^-1), among others, depending on the ketone’s structure.