How To Write Agcl Formula? Simple Explanation
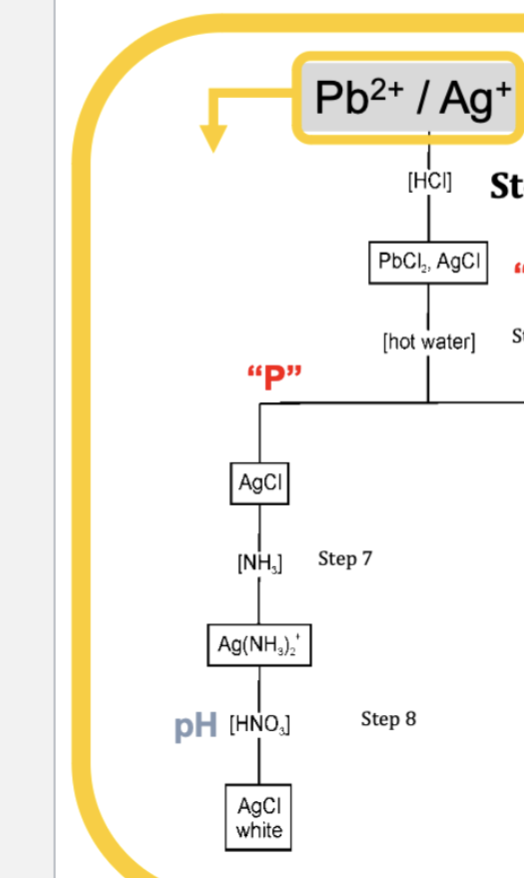
To write the formula for Silver Chloride, which is commonly denoted as AgCl, we need to understand the basic principles of chemical formula writing. Chemical formulas represent the type and number of atoms present in the compound’s molecule. Here’s a straightforward explanation of how to write the AgCl formula:
Identify the Elements: The first step is to identify the elements involved. In this case, we are dealing with Silver (Ag) and Chlorine (Cl).
Determine the Valences: Next, we need to know the valences (or charges) of these elements. Silver has a valence of +1, and Chlorine has a valence of -1.
Balance the Charges: To form a neutral compound, the positive and negative charges must be balanced. Since Silver has a +1 charge and Chlorine has a -1 charge, it takes one of each to balance the charges. Therefore, the formula reflects a 1:1 ratio of Silver to Chlorine.
Write the Formula: With the information from the steps above, we can now write the formula. The symbol for Silver is Ag, and the symbol for Chlorine is Cl. Since we need one of each to balance the charges, the formula is AgCl.
Explanation of the Formula: The AgCl formula tells us that one atom of Silver (Ag) combines with one atom of Chlorine (Cl) to form one molecule of Silver Chloride. This compound is often used in various applications, including photography, due to its light-sensitive properties.
Key Points to Remember: - Silver (Ag) has a +1 valence. - Chlorine (Cl) has a -1 valence. - The combination of these elements in a 1:1 ratio forms a neutral compound, which is represented by the formula AgCl.
Writing chemical formulas like AgCl involves understanding the elements’ symbols, their valences, and how they combine in simple ratios to form compounds. This basic knowledge is essential for more complex chemical formula writing and understanding chemical reactions.


