Ch4 Intermolecular Force

The realm of intermolecular forces is a fascinating domain that underlies the behavior of molecules in various states of matter. Intermolecular forces, also known as van der Waals forces, are the attractive and repulsive forces that arise between molecules due to the interactions between their electrons. These forces play a crucial role in determining the physical properties of substances, such as melting and boiling points, viscosity, and solubility.
To comprehend the nature of intermolecular forces, it is essential to delve into the underlying principles of molecular structure and electron distribution. Molecules are composed of atoms that are bonded together through covalent bonds, which involve the sharing of electron pairs between atoms. However, the distribution of electrons within a molecule is not always symmetrical, leading to the formation of partial charges. These partial charges, in turn, give rise to intermolecular forces.
There are several types of intermolecular forces, each with distinct characteristics and strengths. The primary types of intermolecular forces are:
- London Dispersion Forces: These forces arise due to the temporary dipoles that form in atoms or molecules. Temporary dipoles are created when electrons are distributed unevenly around an atom or molecule, resulting in a brief moment of partial positive and negative charges. London dispersion forces are the weakest of the intermolecular forces and are responsible for the attraction between non-polar molecules.
- Dipole-Dipole Forces: These forces occur between polar molecules, which have a permanent separation of charge. The positive end of one molecule is attracted to the negative end of another molecule, resulting in a stronger force than London dispersion forces.
- Hydrogen Bonding: Hydrogen bonding is a special type of dipole-dipole force that occurs when a hydrogen atom is bonded to a highly electronegative atom, such as oxygen, nitrogen, or fluorine. Hydrogen bonds are stronger than London dispersion forces and dipole-dipole forces, and are responsible for the high boiling points of substances like water and ammonia.
- Ion-Dipole Forces: These forces arise between ions and polar molecules. Ion-dipole forces are stronger than London dispersion forces and dipole-dipole forces, and are responsible for the interaction between ions and molecules in solutions.
The strength and nature of intermolecular forces have a profound impact on the physical properties of substances. For example:
- Melting and Boiling Points: The strength of intermolecular forces determines the energy required to overcome the attractive forces between molecules, thereby influencing the melting and boiling points of substances.
- Viscosity: The viscosity of a substance is influenced by the strength of intermolecular forces, with stronger forces resulting in higher viscosity.
- Solubility: The solubility of a substance in a solvent is influenced by the nature and strength of intermolecular forces between the substance and the solvent molecules.
In conclusion, intermolecular forces play a vital role in determining the physical properties of substances. Understanding the nature and strength of these forces is essential for predicting the behavior of molecules in various states of matter.
It's worth noting that intermolecular forces are responsible for many of the unique properties of water, such as its high surface tension and boiling point. The hydrogen bonding between water molecules is particularly strong, which is why water is essential for many biological and chemical processes.
Physical Properties Influenced by Intermolecular Forces
The following table illustrates the physical properties that are influenced by intermolecular forces:
| Physical Property | Influence of Intermolecular Forces |
|---|---|
| Melting Point | Strength of intermolecular forces determines the energy required to overcome attractive forces between molecules |
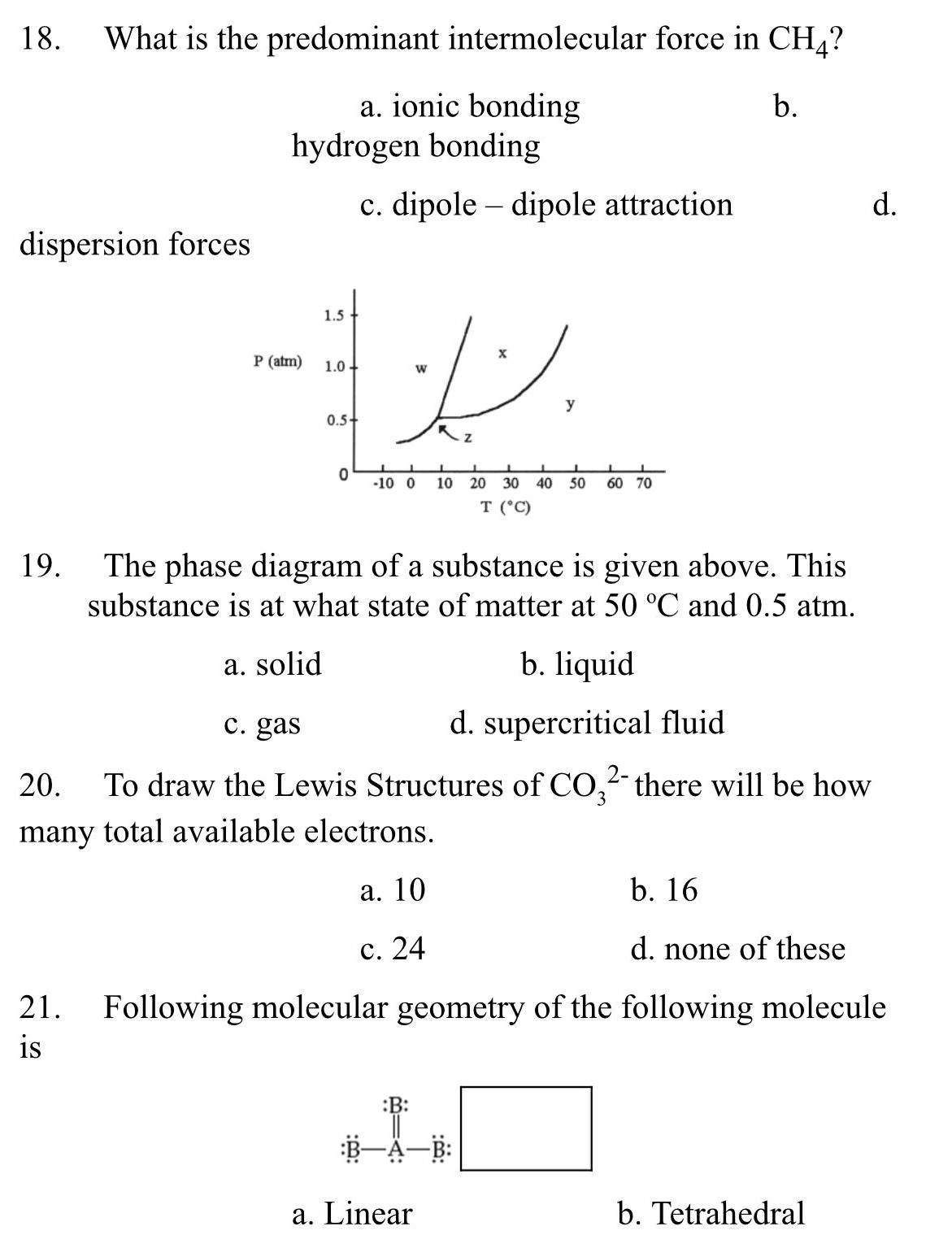
| Boiling Point | Strength of intermolecular forces determines the energy required to overcome attractive forces between molecules |
| Viscosity | Strength of intermolecular forces influences the resistance to flow |
| Solubility | Nature and strength of intermolecular forces between substance and solvent molecules influence solubility |
Real-World Applications of Intermolecular Forces
The understanding of intermolecular forces has numerous real-world applications, including:
- Developing New Materials: The design of new materials with specific properties, such as high-strength polymers or self-healing materials, relies on the understanding of intermolecular forces.
- Improving Pharmaceutical Formulations: The interaction between pharmaceutical molecules and their solvents is influenced by intermolecular forces, which can affect the efficacy and stability of the formulation.
- Enhancing Energy Efficiency: The development of more efficient energy storage and conversion systems, such as batteries and fuel cells, relies on the understanding of intermolecular forces.
What is the difference between London dispersion forces and dipole-dipole forces?
+Why are hydrogen bonds stronger than London dispersion forces and dipole-dipole forces?
+Hydrogen bonds are stronger due to the high electronegativity of the atom bonded to the hydrogen, which results in a more significant partial positive charge on the hydrogen atom, leading to a stronger attraction between molecules.
In summary, intermolecular forces play a vital role in determining the physical properties of substances and have numerous real-world applications. The understanding of these forces is essential for the development of new materials, improvement of pharmaceutical formulations, and enhancement of energy efficiency.
