12 Sodium Acetate Trihydrate Formulas For Easy Learning
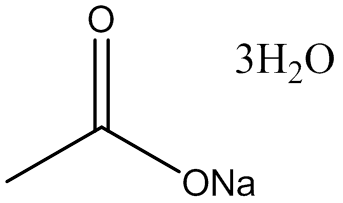
Sodium acetate trihydrate, a compound with the chemical formula CH₃COONa·3H₂O, is a vital substance in various chemical and biological applications. Understanding its properties, synthesis, and uses can be quite intricate, but breaking down its formula and related chemical reactions can simplify the learning process. Here are 12 Sodium Acetate Trihydrate formulas and related concepts to enhance easy learning:
Chemical Formula: CH₃COONa·3H₂O - This formula represents sodium acetate trihydrate, indicating one molecule of sodium acetate (CH₃COONa) combined with three water molecules (3H₂O).
Molecular Weight Calculation: To calculate the molecular weight of sodium acetate trihydrate, you need to sum the atomic weights of all atoms in the molecule. The formula for this calculation is:
- Molecular Weight = (12.01 for C) + (3*1.008 for H) + (12.012 for two O in acetate) + (22.99 for Na) + (3(2*1.008 + 16.00) for 3H₂O)
- Simplified: Molecular Weight = 12.01 + 3.024 + 24.02 + 22.99 + 3*(2.016 + 16.00)
- Further Simplified: Molecular Weight = 12.01 + 3.024 + 24.02 + 22.99 + 3*18.016
- Calculation: Molecular Weight = 12.01 + 3.024 + 24.02 + 22.99 + 54.048
- Final Calculation: Molecular Weight = 116.103 g/mol
Synthesis Equation: The synthesis of sodium acetate trihydrate from glacial acetic acid and sodium hydroxide can be represented as:
- CH₃COOH + NaOH → CH₃COONa + H₂O
- To form the trihydrate, the sodium acetate is then crystallized in water:
- CH₃COONa + 3H₂O → CH₃COONa·3H₂O
Dissociation Equation: When sodium acetate trihydrate dissolves in water, it dissociates into its constituent ions:
- CH₃COONa·3H₂O → Na⁺ + CH₃COO⁻ + 3H₂O
pH Calculation: Sodium acetate is a salt of a weak acid (acetic acid) and a strong base (sodium hydroxide), making its solution slightly basic. The pH can be calculated using the Henderson-Hasselbalch equation:
- pH = pKa + log([A⁻]/[HA])
- For sodium acetate, pKa of acetic acid is approximately 4.76, and [A⁻] = [CH₃COO⁻], [HA] = [CH₃COOH]. In a solution of sodium acetate trihydrate, [CH₃COOH] is very small compared to [CH₃COO⁻], making the solution basic.
Buffer Solution: Sodium acetate can form a buffer solution with acetic acid, useful for maintaining stable pH in various chemical reactions:
- Equation: CH₃COONa + CH₃COOH ⇌ (CH₃COO⁻ + H⁺) + (CH₃COO⁻ + Na⁺)
- Simplified Concept: The acetate ion (CH₃COO⁻) from sodium acetate and the acetic acid (CH₃COOH) can resist pH change by shifting the equilibrium.
Heat of Crystallization: When sodium acetate trihydrate is heated, it loses water of crystallization:
- CH₃COONa·3H₂O → CH₃COONa + 3H₂O
- This process involves the heat of crystallization, which is the energy change associated with the formation or dissolution of a crystal lattice.
Solubility Product Constant (Ksp): The solubility of sodium acetate trihydrate in water can be described by its Ksp, indicating the equilibrium between the dissolved ions and the solid compound:
- Ksp = [Na⁺][CH₃COO⁻]
Van ’t Hoff Factor: The van ’t Hoff factor (i) is used to predict the effect of a solute on the colligative properties of a solution. For sodium acetate trihydrate, which completely dissociates into two ions:
- i = 2 (for the ions Na⁺ and CH₃COO⁻)
Boiling Point Elevation: Sodium acetate trihydrate can cause a boiling-point elevation in a solution due to the dissociation of the salt into ions, which increases the boiling point of the solvent:
- ΔT = iKb × m
- Where ΔT is the change in boiling point, i is the van ’t Hoff factor, Kb is the boiling-point elevation constant, and m is the molality of the solution.
Freezing Point Depression: Similarly, the freezing point of a solution containing sodium acetate trihydrate will be lowered due to the presence of dissolved ions:
- ΔT = iKf × m
- Where ΔT is the change in freezing point, i is the van ’t Hoff factor, Kf is the freezing-point depression constant, and m is the molality of the solution.
Osmotic Pressure: The osmotic pressure of a solution of sodium acetate trihydrate can be calculated using the formula:
- π = iMRT
- Where π is the osmotic pressure, i is the van ’t Hoff factor, M is the molarity of the solution, R is the gas constant, and T is the temperature in Kelvin.
These formulas and concepts are essential for understanding the properties and behaviors of sodium acetate trihydrate, facilitating a deeper and more comprehensive learning experience in chemistry and related fields.

