10+ Molar Mass Secrets For Calcium Fluoride Mastery
Calcium fluoride, a compound with the chemical formula CaF2, is a crucial material in various industrial and medical applications. Understanding its properties, particularly its molar mass, is essential for professionals and students alike. The molar mass of a substance is a fundamental concept in chemistry, representing the mass of one mole of that substance. In this article, we will delve into the world of calcium fluoride, exploring its molar mass, properties, and applications, while uncovering secrets that can help you master this compound.
Introduction to Calcium Fluoride
Calcium fluoride is an ionic compound composed of calcium cations (Ca2+) and fluoride anions (F-). It occurs naturally as the mineral fluorite, which is the primary source of fluorine. The compound has a wide range of applications, including in the production of hydrofluoric acid, fluoropolymers, and as a flux in the steel industry. Its unique optical properties make it an essential component in the manufacture of telescopes and other optical instruments.
Understanding Molar Mass
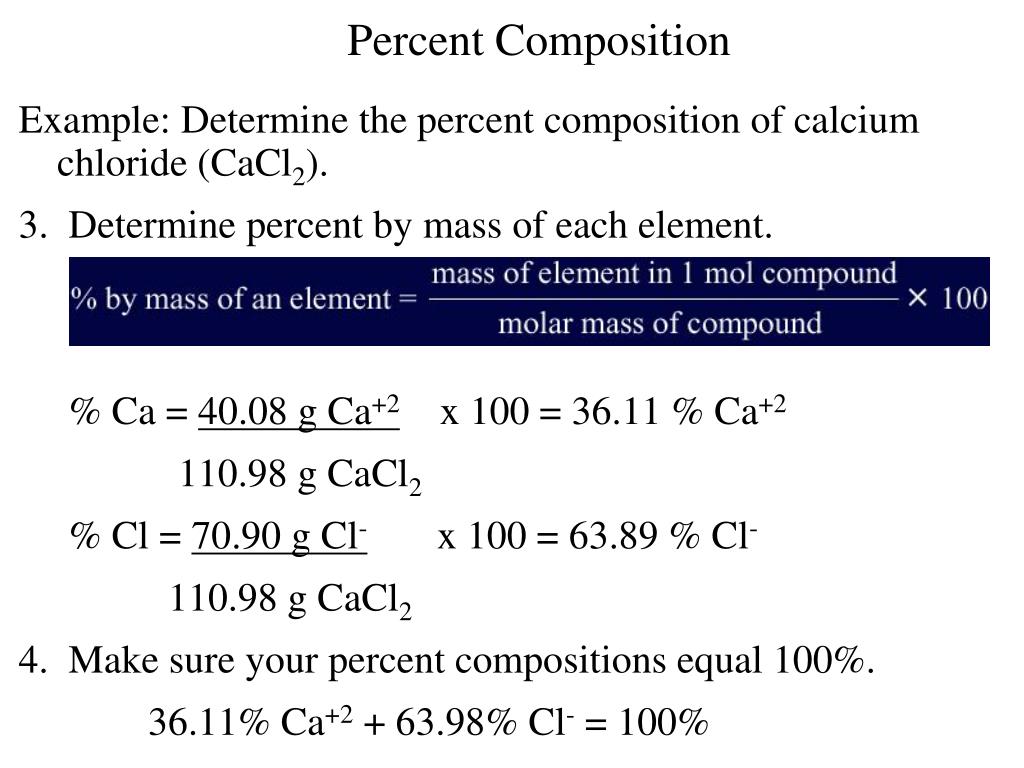
The molar mass of a compound is calculated by summing the atomic masses of its constituent atoms. For calcium fluoride, the calculation involves adding the atomic mass of one calcium atom to twice the atomic mass of fluorine, since there are two fluorine atoms in the compound. The atomic mass of calcium is approximately 40.078 g/mol, and the atomic mass of fluorine is about 18.998 g/mol.
Calculating the Molar Mass of Calcium Fluoride
To calculate the molar mass of CaF2, we follow the formula:
Molar Mass of CaF2 = Atomic Mass of Ca + 2 * Atomic Mass of F
Substituting the known values:
Molar Mass of CaF2 = 40.078 g/mol + 2 * 18.998 g/mol Molar Mass of CaF2 = 40.078 g/mol + 37.996 g/mol Molar Mass of CaF2 = 78.074 g/mol
Thus, the molar mass of calcium fluoride is approximately 78.074 g/mol.
Secrets to Mastering Calcium Fluoride
Understanding Its Crystal Structure: Calcium fluoride has a cubic crystal structure, which contributes to its unique optical and electrical properties. Mastering the crystal structure can help in understanding its behavior in various applications.
Applications in Medicine: Calcium fluoride is used in dental applications to prevent tooth decay. It is also a component in some pharmaceuticals. Understanding its role in medicine can provide insights into its biochemical interactions.
Industrial Applications: The compound is crucial in the steel industry as a flux to remove impurities. It’s also used in the manufacture of glass and ceramics. Knowledge of these applications can highlight its economic importance.
Optical Properties: Calcium fluoride has excellent optical properties, making it ideal for use in telescopes, microscopes, and other optical instruments. Its transparency over a wide range of wavelengths is particularly valuable.
Chemical Reactivity: Understanding the chemical reactivity of calcium fluoride, including its reactions with acids and bases, can provide insights into its handling and storage.
Environmental Impact: The extraction and processing of calcium fluoride have environmental implications. Understanding these can help in developing sustainable practices.
Advanced Materials: Research into calcium fluoride and its compounds has led to the development of advanced materials with unique properties. Exploring these can reveal future applications and technologies.
Biological Effects: While calcium fluoride has beneficial applications, its biological effects, particularly at high concentrations, need to be understood to ensure safe handling and use.
Synthesis Methods: Mastering the synthesis methods of calcium fluoride, including its purification, can be crucial for achieving high-quality material for specific applications.
Future Research Directions: Looking into future research directions, such as its use in nanotechnology and biomedical engineering, can provide a glimpse into the potential of calcium fluoride to solve complex problems.
Conclusion
Calcium fluoride is a compound of immense industrial and medical importance. Its molar mass, calculated to be approximately 78.074 g/mol, is a fundamental property that underpins its applications. By uncovering the secrets of calcium fluoride, from its crystal structure and optical properties to its industrial and medical applications, professionals and students can deepen their understanding of this versatile compound. Whether in the context of material science, chemistry, or environmental studies, mastery of calcium fluoride can open doors to new technologies, applications, and innovations.
FAQ Section
What is the molar mass of calcium fluoride?
+The molar mass of calcium fluoride (CaF2) is approximately 78.074 g/mol, calculated by adding the atomic mass of calcium to twice the atomic mass of fluorine.
What are the primary applications of calcium fluoride?
+Calcium fluoride has a wide range of applications, including in the production of hydrofluoric acid, fluoropolymers, as a flux in the steel industry, and in the manufacture of optical instruments due to its unique optical properties.
How is calcium fluoride used in medicine?
+In medicine, calcium fluoride is used in dental applications to prevent tooth decay. It is also a component in some pharmaceuticals, highlighting its role in biochemical interactions and its potential for further medical applications.
What are the environmental implications of extracting and processing calcium fluoride?
+The extraction and processing of calcium fluoride have environmental implications, including the potential for pollution and habitat disruption. Understanding these implications is crucial for developing sustainable practices and minimizing its ecological footprint.
In conclusion, the journey to mastery of calcium fluoride involves understanding its molar mass, properties, and applications, as well as exploring its potential for future innovations. By uncovering its secrets and addressing the complexities of its use, we can unlock new possibilities and ensure a sustainable future for its applications.


